
Application of Gibbs Rule to VLE YouTube
chrome_reader_mode Entry Reader Switch. { }.

Phase Diagrams Gibbs Phase Rule (w/ 5 Examples) YouTube
The Gibbs phase-rule for a non-reacting system is given by, (1) where f is the number of degrees of freedom, N the number of components and the number of phases. Clearly, for an unary system ( ) with two phases in equilibrium ( = 2) there is only one degree of freedom, which shows that the NVT -Gibbs is the only eligible ensemble for pure.

Gibbs Phase Rule Gibbs Phase Rule And It's Derivation4Phase
The Gibbs phase rule for a multicomponent system to be described in Sec. 13.1 shows that a two-component, two-phase system at equilibrium has only two independent intensive variables. Thus at a given temperature and pressure, the mole fraction compositions of both phases are fixed; the compositions depend only on the identity of the substances.
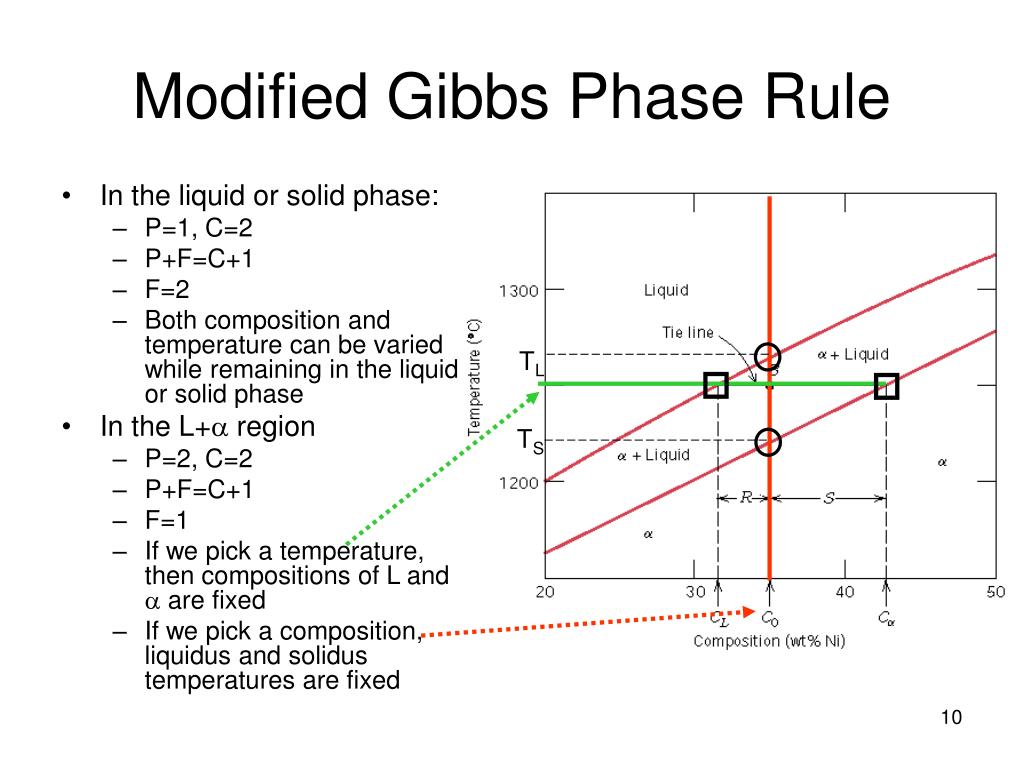
PPT Chapter 9 PowerPoint Presentation, free download ID4271442
1. Introduction. Just a few years after Andrews' experimental discovery of the critical opalescence in carbon dioxide (which Andrews himself coined as the critical point) [], Gibbs introduced the phase rule [2,3].The so-called Gibbs phase rule (GPR) is solely based on general thermodynamics arguments and can give a remarkable account of the possible number of phases that can coexist for a.

Thermodynamics What is Gibbs phase rule? YouTube
It then discusses phase diagrams for some representative types of multicomponent systems, and shows how they are related to the phase rule and to equilibrium concepts developed in Chapters 11 and 12. 13.1: The Gibbs Phase Rule for Multicomponent Systems
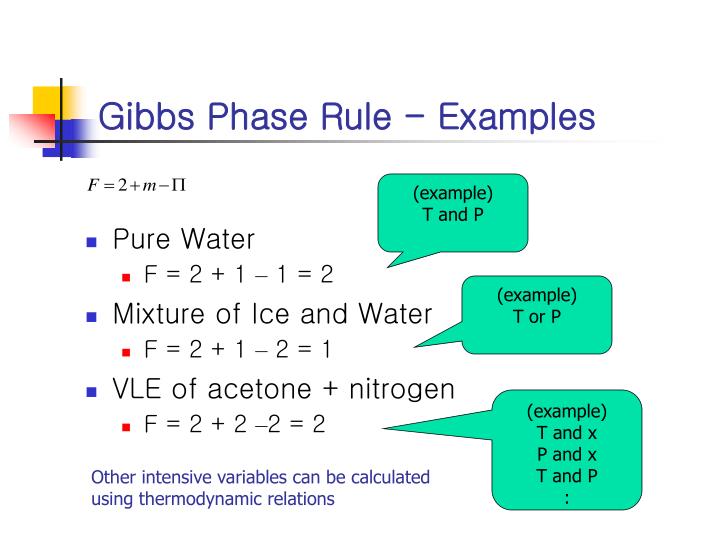
PPT Chapter 6. Multiphase Systems PowerPoint Presentation ID920989
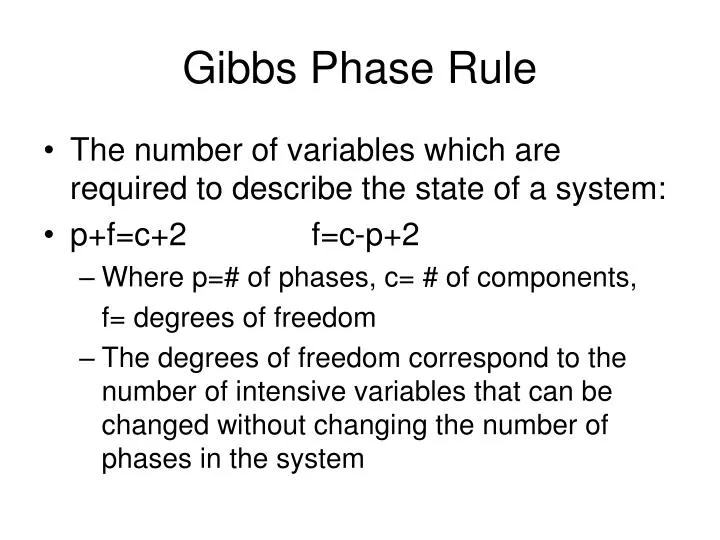
Definitions. Gibbs Phase Rule is expressed by the simple formulation: P + F = C + 2, where. P is the number of phases in the system. A phase is any physically separable material in the system. Every unique mineral is a phase (including polymorphs); igneous melts, liquids (aqueous solutions), and vapor are also considered unique phases.

Lec 62 The Gibbs Phase Rule YouTube
13.1: The Gibbs Phase Rule for Multicomponent Systems. In Sec. 8.1.7, the Gibbs phase rule for a pure substance was written F = 3 − P F = 3 − P. We now consider a system of more than one substance and more than one phase in an equilibrium state. The phase rule assumes the system is at thermal and mechanical equilibrium.
13.1 The Gibbs Phase Rule for Systems
Physics 127b: Statistical Mechanics Phase Transitions in Multicomponent Systems The Gibbs Phase Rule Consider a system with ncomponents (different types of molecules) with rphases in equilibrium. The state of each phase is defined by P;Tand then.n−1/concentration variables in each phase. The phase equilibrium at given P;Tis defined by the equality of nchemical potentials between
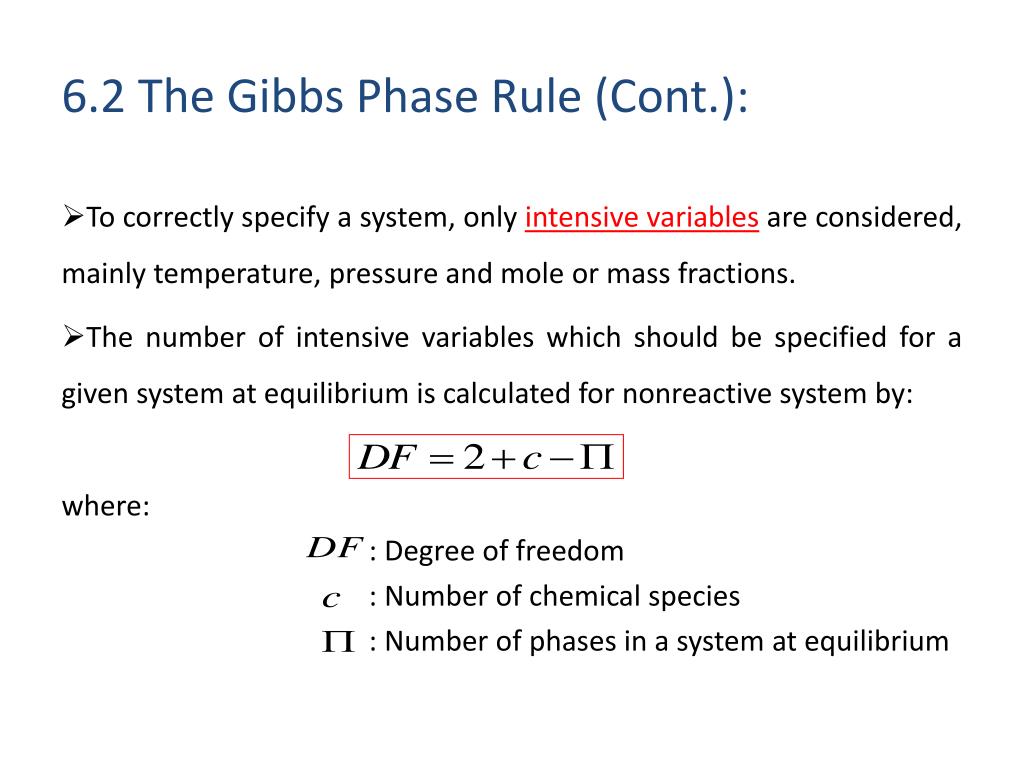
PPT CHE201 Introduction to Chemical Engineering PowerPoint
Equation ( 27.19) is the Gibbs Phase Rule (GPR) and was originally derived by J.W. Gibbs in 1875. Note that if there are additional constraints (e.g., chemical reactions), L is decreased further by the number of additional constraints, r, that is, L = n + 2 − π − r.

and Multiphase Systems Phase Rule Thermodynamics
Here, G (i) (x (i)) describes the molar Gibbs free energy of a phase i ∈ {1,., N} (phase specific Gibbs function), consisting of a reference part G ref (i), an ideal part G id (i), and an excess part G ex (i) describing the deviation of the real mixture from the ideal one. More details are given in the appendix. 2.2 Stoichiometric Constraints. Phases and phase constituents are formed.

phase equilibrium — Computational Thermodynamics
\( \newcommand{\subs}[1]{_{\text{#1}}} % subscript text\) \( \newcommand{\sups}[1]{^{\text{#1}}} % superscript text\) \( \newcommand{\st}{^\circ} % standard state.

The Gibbs Phase Rule Semantic Scholar...The Gibbs Phase Rule! • The
Gibbs Phase Rule. In any one-component system with three phases, there is only one point at which all three phases coexist in equilibrium. This is known as the triple point.Although the Gibbs free energy \(G\) is a function of \(n\), \(P\), and \(T\), \(G = G(n, P, T)\), if we allow ourselves to treat \(P\) and \(T\) as experimental control parameters, as is done when we construct a phase.
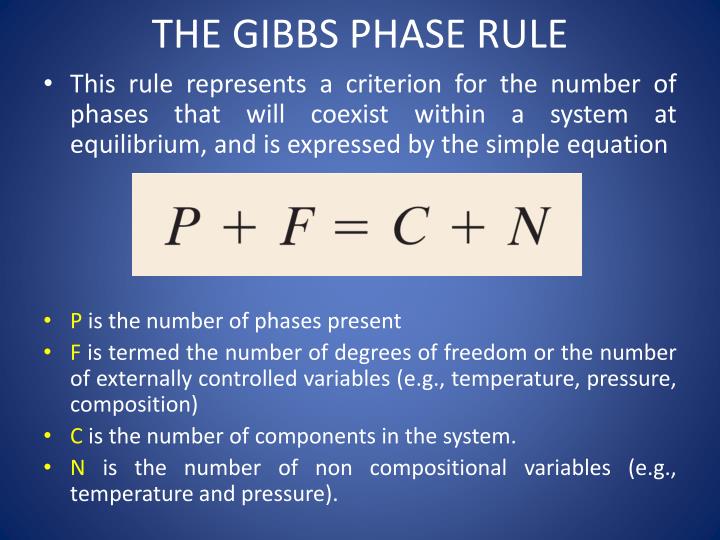
PPT Phase Diagram PowerPoint Presentation ID6013819
13.1 THE GIBBS PHASE RULE FOR MULTICOMPONENT SYSTEMS In Sec. 8.1.7, the Gibbs phase rule for a pure substance was written F D 3 P.Wenow consider a system of more than one substance and more than one phase in an equilibrium state. The phase rule assumes the system is at thermal and mechanical equilibrium.
PPT Gibbs Phase Rule PowerPoint Presentation, free download ID6530794
Gibbs Phase Rule. Gibbs phase rule states that if the equilibrium in a heterogeneous system is not affected by gravity or by electrical and magnetic forces, the number of degrees of freedom is given by the equation. F=C-P+2. where, C is the number of chemical components, P is the number of phases. Basically, it describes the mathematical.

Gibb's Phase rule, Definition, and Derivation Chemistry Notes
The application of the phase rule to multicomponent systems will be taken up in Sec. 13.1. Equation 8.1.17 is a special case, for \(C = 1\), of the more general Gibbs phase rule \(F = C - P + 2\). We may interpret the variance \(F\) in either of two ways:

Gibbs Phase Rule (हिंदी) YouTube
It is in contrast to Gibb's phase rule and Duhem's theorem for open and closed systems, respectively. The Gibbs phase rule is incorporated into the present formulation.. Extended phase rule for non-reactive, multiphase, multicomponent chemical systems. Fluid Phase Equilibria, 58: 13 345. A comprehensive phase rule has been developed for non.